CBI comments on proposals for medicines coming into NI
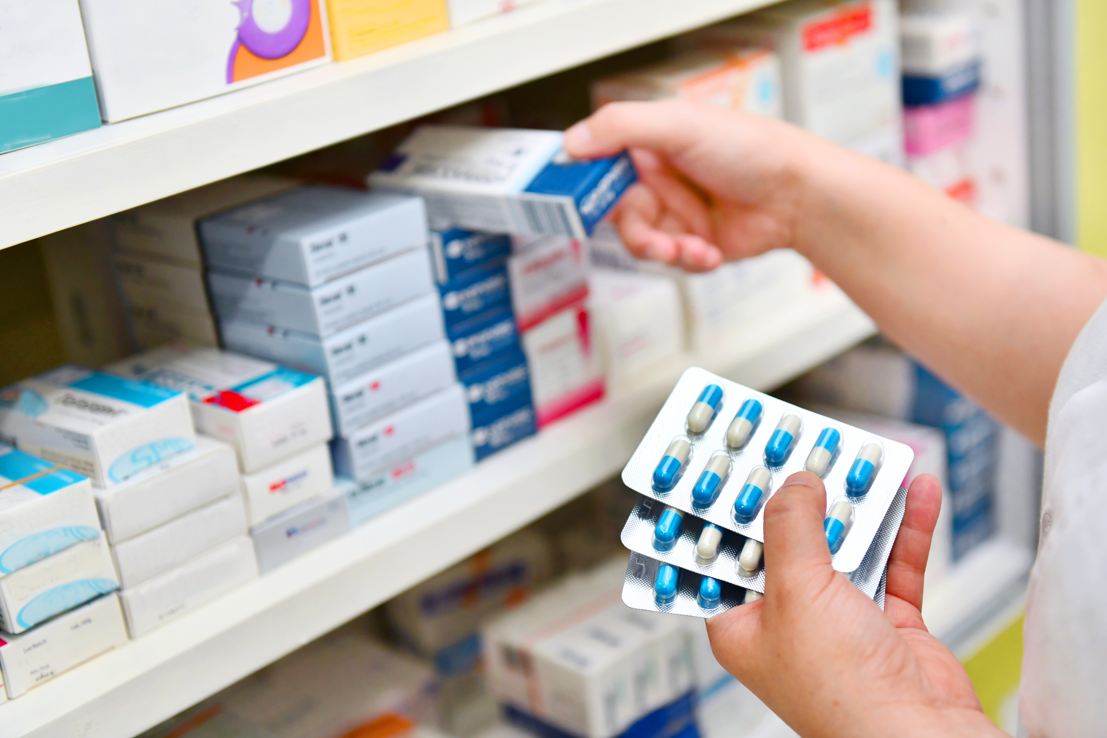
The CBI has commented on an EU commitment that medicines entering Northern Ireland from Great Britain will not need additional labelling or testing.
Medicines will continue to be available in Northern Ireland at the same time as in the rest of the UK, the European Union (EU) has said.
The protocol means Northern Ireland is still inside the EU’s pharmaceutical regulatory system, but it gets most of its medicines from Great Britain, which is not.
The EU says its new proposals mean medicines entering NI from GB will not need additional labelling or testing.
Sean McGuire, CBI Europe Director, said: “The CBI and wider industry have been calling for greater certainty on the movement of medicines between GB and NI – today’s announcement from the EU looks like a welcome step in that direction.
“Industry will now examine the detail on this complex and sensitive issue.
“Businesses urge both sides to work together and agree lasting, durable solutions to unlock the remaining issues with the Protocol.”
European Commission Vice-President Maroš Šefčovič said a commitment to ensure the supply of medicines into Northern Ireland was being turned into “a lasting solution”.
He said the protocol “has the flexibility to work on the ground”.
For generic drugs like paracetamol, the UK regulator can continue to approve drugs for NI and companies in GB can continue to use the the same pack and leaflet for all parts of the UK, with no need for NI-specific packaging.
All regulatory functions, like batch testing, will remain wherever they are now in the UK – with no need to relocate any testing facilities from GB to NI.
For new medicines, like cancer drugs, any product authorised in the UK can be supplied to NI, until the relevant authorisation is also given in the EU.
The EU says this “bridging solution” is in addition to the existing compassionate and emergency use early access mechanisms under EU law.
For all types of medicines, no manufacturing authorisation or import licence will be required for bringing medicines into NI from the rest of the UK.
In addition, EU medicine unique identifiers won’t have to be removed from products transiting through GB to NI and the reaffixed when entering NI.
However this derogation is only for three years, which the EU says will allow more time for industry to adapt.